(Photo by Karola G via Pexels)
By Stephen Beech
Stress may lead to altered blood flow in the brain, reveals a new study.
The findings may give vital clues to the exact causes of neurodegenerative brain diseases such as Alzheimer's and dementia, say scientists.
One key characteristic in the brains of patients with such diseases is reduced blood flow.
Now, researchers at Pennsylvania State University have found that a rare neuron that is "extremely vulnerable" to anxiety-induced stress appears to be responsible for regulating blood flow and coordinating neural activity in mice.
The Penn State team found that eliminating type-one nNOS neurons - which make up less than 1% of the brain’s 80 billion neurons and die off when exposed to too much stress - resulted in a drop in both blood flow and electrical activity in mice brains.
They say their findings, published in the journal eLife, show the impact this neuron type has on the proper brain functions of animals, including humans.
Principal investigator Professor Patrick Drew explained that although more than 20 different types of neurons make up any section of the brain, type-one nNOS neurons in the somatosensory cortex - the region that processes touch, temperature and other sensory input from the body - play a "critical" role in stimulating the "spontaneous oscillation” of arteries and veins in the brain.

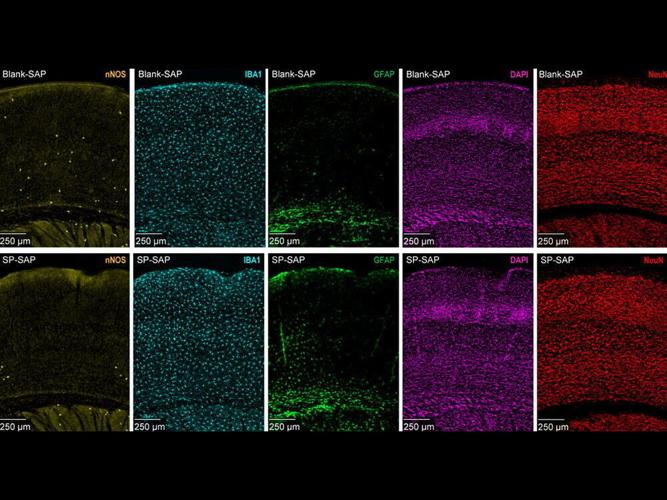
The type of neuron the team targeted, type-one nNOS, which is colored yellow in the figure above, is rare compared to other neurons in the brain. (Patrick Drew/Penn State via SWNS)
He said: “In your brain, arteries, veins, and capillaries help move fluid around by constantly dilating and constricting every few seconds, which we call spontaneous oscillation.
“Previous work from our lab has shown that nNOS neurons are important for regulating blood flow in the brain.
"After targeting and eliminating a subset of these neurons, we observed a significant reduction in the amplitude of these oscillations.”
Drew explained that when mice are exposed to mentally stressful experiences, the delicate neurons can easily die.
While other researchers have previously connected ageing with reduced brain performance and increased risk to neurodegenerative diseases, Drew says there is much less research on stress and the negative impacts it can have on blood flow.
He said: “We are broadly interested in how blood flow is regulated in the brain, as it supplies nutrients and oxygen to neurons.

(Photo by Mental Health America (MHA) via Pexels)
“Reduced blood flow is one of many contributing factors to reduced brain function and neurodegenerative diseases.
"While we know ageing plays a major role in this, losing these rare neurons to chronic stress could be an unexplored environmental cause for poor brain health.”
To understand what happens without type-one nNOS neurons in the brain, the team injected mice with a mix of saporin - a toxic protein capable of killing neurons - and a chemical chain of amino acids known as a peptide, which can identify and latch onto specific genetic markers emitted by type-one nNOS neurons.
The markers differentiate type-one nNOS neurons in the brain, allowing the researchers to systematically deliver saporin and eliminate them without harming other neurons.
The Penn State team is the first to use that method to target those specific neurons, according to Drew.
He added: "While a mouse brain isn’t a perfect model for the human brain, much of the physiology - including neuronal type and composition - match, so this type of work can reveal information that likely maps to humans."
After injecting the mice, the researchers recorded changes in brain activity and physical behaviour such as eye dilation and whisker movement.

(Photo by Anna Tarazevich via Pexels)
The team observed cerebral blood vessel oscillations at micrometre-level resolution - around 100 times smaller than the width of a human hair.
They also used electrodes and advanced imaging to track electrical currents in the brain.
Drew said: "The mice showed not just reduced blood flow, but weaker neural activity across the brain, indicating that these type-one nNOS neurons seem to be important in helping neurons communicate with one another."
The team also identified the reductions in blood flow and neural activity were higher during sleep than in the awake state, indicating the neurons could play a role in supporting the brain during sleep.
Drew said: "Optimizing this procedure will provide an efficient and non-genetic way for researchers to study type-one nNOS neurons and the impacts of losing them in further detail."
Although it's too early to draw a direct connection between reduced density of the neurons with increased risk of Alzheimer’s and dementia, he says future research will focus on investigating how the loss of the neurons interacts with genetic risk factors for the diseases.